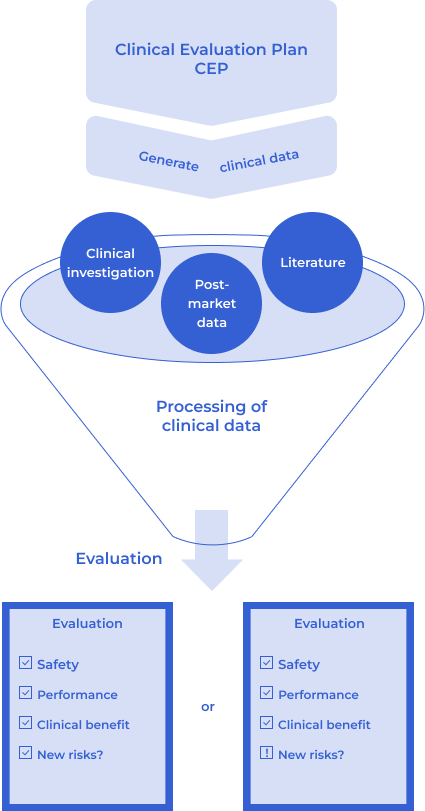
Clinical data
Clinical Data for Clinical Evaluation
1. Scientific Literature
The systematic conduct of a scientific literature search is a carefully planned process. It not only involves extracting data from appropriate sources and assessing their relevance to the clinical evaluation, but also includes a critical appraisal of the methodological quality of the literature. An appropriate weighting of the evidence is essential to ensure a reliable evidence base.
Product-specific scientific literature searches in medical journals (e.g., via PubMed, Embase, and other databases), specialist books, and product registries can be used to identify clinical data. These data may relate to the device under evaluation or to devices that have been demonstrated to be equivalent.
2. Clinical Investigations
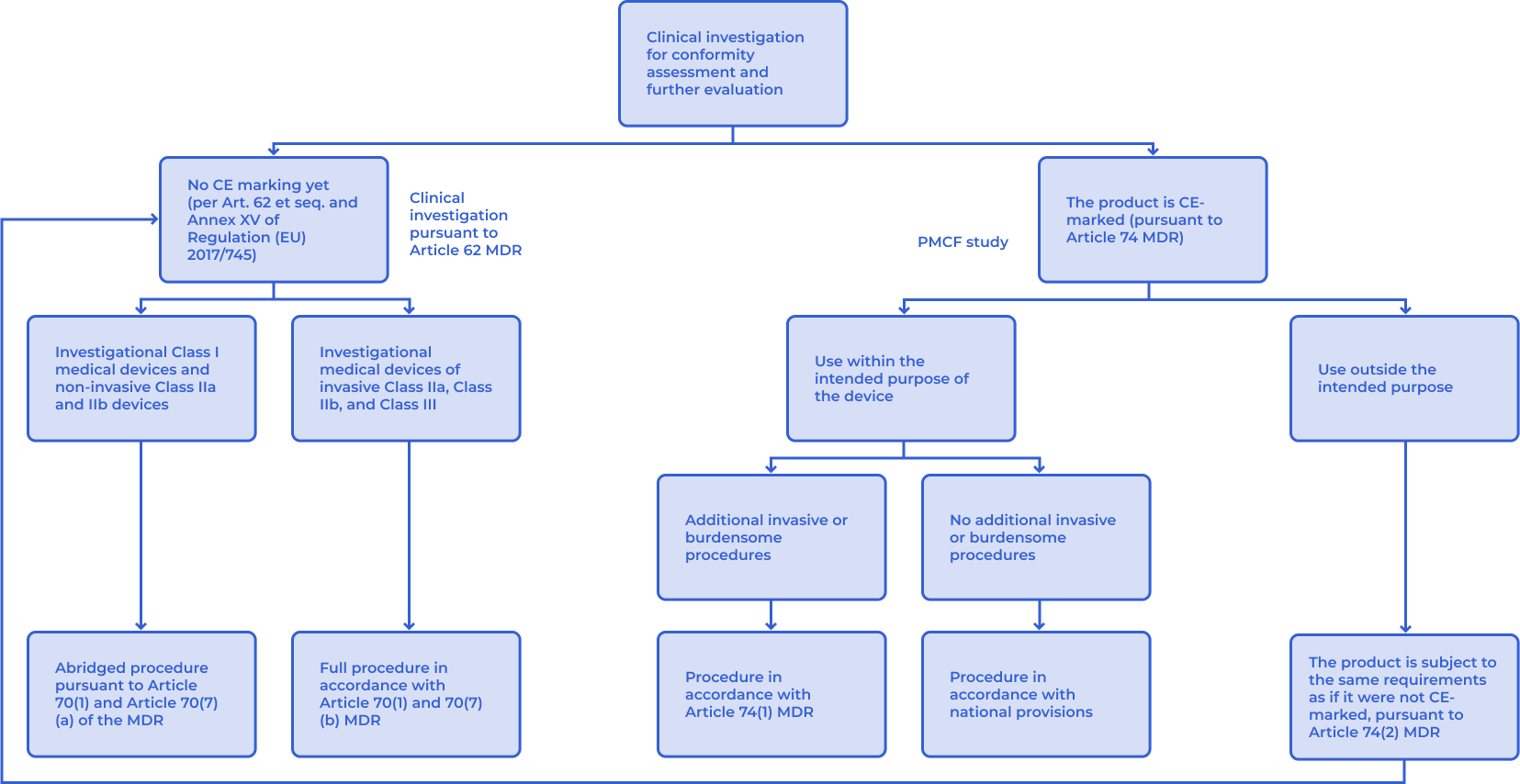
The need for a clinical investigation is typically identified during the clinical evaluation. If gaps in clinical data are revealed ‒ i.e., if data are insufficient in quantity or quality ‒ they must be generated through clinical investigations.
The type of clinical investigation required depends largely on whether the medical device is CE-marked, whether it is used within its intended purpose, and whether any additional invasive or burdensome procedures will be applied to participants.
In the context of conformity assessment or further product evaluation, two main types of clinical investigations are defined under Regulation (EU) 2017/745 (MDR):
- Clinical investigations acc. to Article 62
- PMCF studies acc. to Article 74
The regulatory decision pathway for determining the appropriate type of clinical investigation is illustrated in the corresponding figure. Whether a clinical investigation requires prior authorisation or notification depends on the type of clinical investigation. However, every clinical investigation must at a minimum comply with the applicable sections of EN ISO 14155 and the General Data Protection Regulation (GDPR), and in most cases, approval from an ethics committee is also required.